What Is The Change In The Atomic Number When An Atom Emits An Alpha Particle
17.iii: Types of Radioactivity: Blastoff, Beta, and Gamma Disuse
- Page ID
- 161974
Learning Objectives
- Compare qualitatively the ionizing and penetration power of alpha particles \(\left( \alpha \correct)\), beta particles \(\left( \beta \right)\), and gamma rays \(\left( \gamma \right)\).
- Express the changes in the atomic number and mass number of a radioactive nuclei when an alpha, beta, or gamma particle is emitted.
- Write nuclear equations for alpha and beta decay reactions.
Many nuclei are radioactive; that is, they decompose past emitting particles and in doing so, become a different nucleus. In our studies up to this indicate, atoms of one element were unable to modify into dissimilar elements. That is considering in all other types of changes discussed, only the electrons were changing. In these changes, the nucleus, which contains the protons that dictate which element an cantlet is, is changing. All nuclei with 84 or more protons are radioactive, and elements with less than 84 protons have both stable and unstable isotopes. All of these elements tin can get through nuclear changes and turn into different elements.
In natural radioactive decay, three common emissions occur. When these emissions were originally observed, scientists were unable to place them as some already known particles so named them:
- alpha particles (\(\alpha \))
- beta particles \(\left( \beta \right)\)
- gamma rays \(\left( \gamma \right)\)
These particles were named using the get-go 3 letters of the Greek alphabet. Some later time, alpha particles were identified as helium-4 nuclei, beta particles were identified as electrons, and gamma rays as a form of electromagnetic radiation like x-rays, except much higher in free energy and even more dangerous to living systems.
The Ionizing and Penetration Power of Radiation
With all the radiations from natural and man-fabricated sources, we should quite reasonably be concerned nigh how all the radiation might affect our health. The damage to living systems is done by radioactive emissions when the particles or rays strike tissue, cells, or molecules and modify them. These interactions tin alter molecular construction and function; cells no longer carry out their proper function and molecules, such as DNA, no longer carry the appropriate information. Large amounts of radiation are very dangerous, even deadly. In most cases, radiation will damage a single (or very small-scale number) of cells by breaking the cell wall or otherwise preventing a cell from reproducing.
The ability of radiation to damage molecules is analyzed in terms of what is called ionizing power. When a radiations particle interacts with atoms, the interaction tin can cause the cantlet to lose electrons and thus become ionized. The greater the likelihood that impairment will occur by an interaction is the ionizing power of the radiation.
Much of the threat from radiation is involved with the ease or difficulty of protecting oneself from the particles. How thick of a wall do you demand to hibernate backside to be condom? The ability of each type of radiation to pass through affair is expressed in terms of penetration power. The more material the radiation can pass through, the greater the penetration power and the more dangerous it is. In full general, the greater mass present, the greater the ionizing power, and the lower the penetration power.
Comparing merely the three common types of ionizing radiation, alpha particles have the greatest mass. Alpha particles have approximately four times the mass of a proton or neutron and approximately 8,000 times the mass of a beta particle. Considering of the large mass of the alpha particle, it has the highest ionizing ability and the greatest ability to harm tissue. That same large size of alpha particles, however, makes them less able to penetrate matter. They collide with molecules very quickly when striking matter, add together ii electrons, and become a harmless helium atom. Alpha particles have the least penetration power and can be stopped past a thick sail of paper or fifty-fifty a layer of clothes. They are also stopped by the outer layer of dead skin on people. This may seem to remove the threat from blastoff particles, simply it is only from external sources. In a nuclear explosion or some sort of nuclear accident, where radioactive emitters are spread effectually in the environment, the emitters can be inhaled or taken in with food or water and one time the alpha emitter is within you, yous have no protection at all.
Beta particles are much smaller than alpha particles and therefore, have much less ionizing power (less ability to damage tissue), but their small size gives them much greater penetration power. Most resource say that beta particles can be stopped by a one-quarter inch thick sheet of aluminum. Once again, however, the greatest danger occurs when the beta emitting source gets inside of you lot.
Gamma rays are not particles, simply a loftier energy form of electromagnetic radiations (like 10-rays, except more powerful). Gamma rays are free energy that has no mass or charge. Gamma rays have tremendous penetration power and require several inches of dense material (like lead) to shield them. Gamma rays may pass all the style through a human body without striking anything. They are considered to take the least ionizing power and the greatest penetration power.
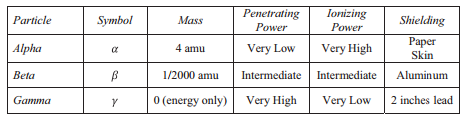
The safest amount of radiation to the human body is null. It is impossible to completely avoid ionizing radiation, so the next best goal is to be exposed to every bit footling as possible. The two best means to minimize exposure are to limit time of exposure, and to increment distance from the source.
Blastoff Decay
The nuclear disintegration procedure that emits alpha particles is called alpha disuse. An instance of a nucleus that undergoes alpha disuse is uranium-238. The blastoff decay of \(\ce{U}\)-238 is
\[\ce{_{92}^{238}U} \rightarrow \ce{_2^4He} + \ce{_{90}^{234}Th} \label{alpha1}\]
In this nuclear change, the uranium cantlet \(\left( \ce{_{92}^{238}U} \correct)\) transmuted into an atom of thorium \(\left( \ce{_{90}^{234}Th} \correct)\) and, in the procedure, gave off an alpha particle. Look at the symbol for the alpha particle: \(\ce{_2^4He}\). Where does an blastoff particle get this symbol? The bottom number in a nuclear symbol is the number of protons. That ways that the blastoff particle has two protons in information technology that were lost past the uranium atom. The two protons also have a charge of \(+2\). The top number, 4, is the mass number or the total of the protons and neutrons in the particle. Because it has ii protons, and a total of four protons and neutrons, blastoff particles must besides have ii neutrons. Alpha particles always have this aforementioned composition: ii protons and ii neutrons.
Some other alpha particle producer is thorium-230.
\[\ce{_{90}^{230}Th} \rightarrow \ce{_2^4He} + \ce{_{88}^{226}Ra} \label{alpha2}\]
These types of equations are called nuclear equations and are like to the chemic equivalent discussed through the previous chapters.
Beta Disuse
Some other common decay process is beta particle emission, or beta disuse. A beta particle is simply a high free energy electron that is emitted from the nucleus. It may occur to yous that we have a logically difficult situation here. Nuclei do non contain electrons and withal during beta decay, an electron is emitted from a nucleus. At the same time that the electron is being ejected from the nucleus, a neutron is becoming a proton. It is tempting to picture this as a neutron breaking into two pieces with the pieces being a proton and an electron. That would exist convenient for simplicity, but unfortunately that is not what happens (more on this subject area will be explained at the end of this department). For convenience, nosotros will care for beta decay every bit a neutron splitting into a proton and an electron. The proton stays in the nucleus, increasing the diminutive number of the atom by one. The electron is ejected from the nucleus and is the particle of radiation chosen beta.
To insert an electron into a nuclear equation and have the numbers add together up properly, an atomic number and a mass number had to be assigned to an electron. The mass number assigned to an electron is zero (0), which is reasonable since the mass number is the number of protons plus neutrons, and an electron contains no protons and no neutrons. The atomic number assigned to an electron is negative one (-1), considering that allows a nuclear equation containing an electron to residue atomic numbers. Therefore, the nuclear symbol representing an electron (beta particle) is
\(\ce{_{-1}^0e}\) or \(\ce{_{-1}^0\beta} \label{beta1}\)
Thorium-234 is a nucleus that undergoes beta disuse. Here is the nuclear equation for this beta decay:
\[\ce{_{ninety}^{234}Th} \rightarrow \ce{_{-one}^0e} + \ce{_{91}^{234}Pa} \label{beta2}\]
Gamma Radiations
Ofttimes, gamma ray production accompanies nuclear reactions of all types. In the alpha decay of \(\ce{U}\)-238, two gamma rays of dissimilar energies are emitted in improver to the alpha particle.
\[\ce{_{92}^{238}U} \rightarrow \ce{_2^4He} + \ce{_{90}^{234}Th} + 2 \ce{_0^0\gamma}\]
Almost all of the nuclear reactions in this affiliate too emit gamma rays, but for simplicity the gamma rays are generally not shown. Nuclear reactions produce a great deal more free energy than chemical reactions. Chemical reactions release the difference between the chemic bond energy of the reactants and products, and the energies released have an society of magnitude of \(1 \times 10^3 \: \text{kJ/mol}\). Nuclear reactions release some of the binding free energy and may convert tiny amounts of matter into energy. The energy released in a nuclear reaction has an order of magnitude of \(one \times 10^{18} \: \text{kJ/mol}\). That means that nuclear changes involve almost one one thousand thousand times more energy per atom than chemical changes!
Notation
Virtually all of the nuclear reactions in this affiliate likewise emit gamma rays, simply for simplicity the gamma rays are generally not shown.
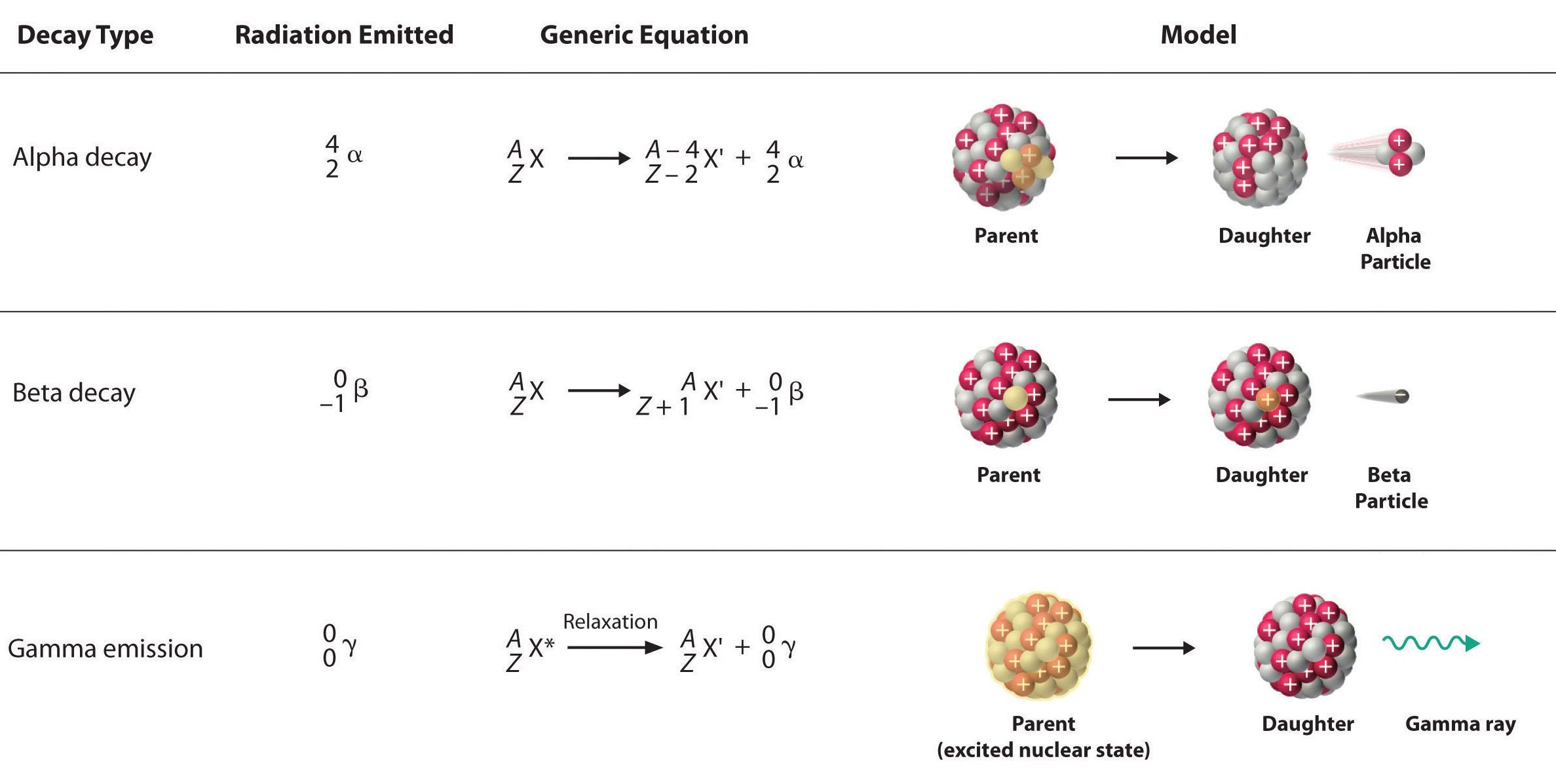
The essential features of each reaction are shown in Figure 17.3.2
Figure 17.3.2: Three near mutual modes of nuclear decay.
"Nuclear Accounting"
When writing nuclear equations, there are some general rules that will assistance yous:
- The sum of the mass numbers (superlative numbers) on the reactant side equal the sum of the mass numbers on the product side.
- The atomic numbers (bottom numbers) on the two sides of the reaction will also be equal.
In the alpha disuse of \(\ce{^{238}U}\) (Equation \(\ref{alpha1}\)), both diminutive and mass numbers are conserved:
- mass number: \(238 = 4 + 234\)
- atomic number: \(92 = 2 + 90\)
Confirm that this equation is correctly balanced past adding up the reactants' and products' diminutive and mass numbers. Besides, note that considering this was an alpha reaction, one of the products is the alpha particle, \(\ce{_2^4He}\).
Notation that both the mass numbers and the atomic numbers add up properly for the beta decay of thorium-234 (Equation \(\ref{beta2}\)):
- mass number: \(234 = 0 + 234\)
- atomic number: \(90 = -1 + 91\)
The mass numbers of the original nucleus and the new nucleus are the aforementioned because a neutron has been lost, merely a proton has been gained, and so the sum of protons plus neutrons remains the same. The diminutive number in the procedure has been increased by 1 since the new nucleus has one more than proton than the original nucleus. In this beta decay, a thorium-234 nucleus has one more than proton than the original nucleus. In this beta disuse, a thorium-234 nucleus has become a protactinium-234 nucleus. Protactinium-234 is also a beta emitter and produces uranium-234.
\[\ce{_{91}^{234}Pa} \rightarrow \ce{_{-1}^0e} + \ce{_{92}^{234}U} \label{nuke1}\]
Once once more, the atomic number increases by one and the mass number remains the same; this confirms that the equation is correctly counterbalanced.
What About Balancing Accuse?
Both alpha and beta particles are charged, simply nuclear reactions in Equations \(\ref{alpha1}\), \(\ref{beta2}\), and most of the other nuclear reactions above, are non balanced with respect to charge, as discussed when balancing redox reactions. When studying nuclear reactions in general, in that location is typically trivial data or business organization about the chemical land of the radioactive isotopes, considering the electrons from the electron deject are non directly involved in the nuclear reaction (in contrast to chemical reactions).
Then information technology is adequate to ignore charge in balancing nuclear reactions, and concentrate on balancing mass and diminutive numbers simply.
Case \(\PageIndex{1}\)
Complete the following nuclear reaction by filling in the missing particle.
\[\ce{_{86}^{210}Rn} \rightarrow \ce{_2^4He} + ?\]
Solution
This reaction is an alpha decay. Nosotros can solve this trouble one of two ways:
Solution 1: When an atom gives off an alpha particle, its atomic number drops by two and its mass number drops by 4, leaving: \(\ce{_{84}^{206}Po}\). Nosotros know the symbol is \(\ce{Po}\), for polonium, because this is the element with 84 protons on the periodic table.
Solution 2: Recall that the mass numbers on each side must total up to the same amount. The same is truthful of the diminutive numbers.
- Mass numbers: \(210 = 4 + ?\)
- Atomic numbers: \(86 = 2 + ?\)
We are left with \(\ce{_{84}^{206}Po}\).
Instance \(\PageIndex{two}\)
Write each of the following nuclear reactions.
a) Carbon-fourteen, used in carbon dating, decays past beta emission.
b) Uranium-238 decays past blastoff emission.
Solution
a) Beta particles accept the symbol \(\ce{_{-ane}^0e}\). Emitting a beta particle causes the atomic number to increase by 1 and the mass number to not change. Nosotros get atomic numbers and symbols for elements using our periodic table. We are left with the post-obit reaction:
\[\ce{_6^{14}C} \rightarrow \ce{_{-1}^0e} + \ce{_7^{fourteen}Northward}\]
b) Alpha particles have the symbol \(\ce{_2^4He}\). Emitting an alpha particle causes the atomic number to subtract by 2 and the mass number to decrease past 4. We are left with:
\[\ce{_{92}^{238}U} \rightarrow \ce{_2^4He} + \ce{_{ninety}^{234}Th}\]
Decay Serial
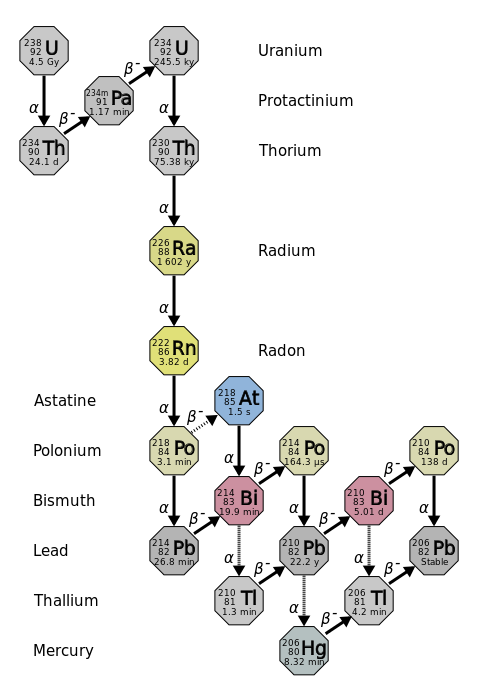
The decay of a radioactive nucleus is a move toward becoming stable. Often, a radioactive nucleus cannot reach a stable country through a unmarried decay. In such cases, a series of decays will occur until a stable nucleus is formed. The decay of \(\ce{U}\)-238 is an example of this. The \(\ce{U}\)-238 decay serial starts with \(\ce{U}\)-238 and goes through fourteen separate decays to finally reach a stable nucleus, \(\ce{Lead}\)-206 (Figure 17.3.three). There are similar decay series for \(\ce{U}\)-235 and \(\ce{Thursday}\)-232. The \(\ce{U}\)-235 series ends with \(\ce{Pb}\)-207 and the \(\ce{Th}\)-232 series ends with \(\ce{Lead}\)-208.
.svg.png?revision=1)
Several of the radioactive nuclei that are found in nature are nowadays there considering they are produced in one of the radioactive disuse series. For example, there may take been radon on the earth at the time of its germination, just that original radon would take all rust-covered by this time. The radon that is present now is present because it was formed in a decay series (mostly by U-238).
Summary
A nuclear reaction is one that changes the structure of the nucleus of an atom. The atomic numbers and mass numbers in a nuclear equation must exist balanced. Protons and neutrons are made upward of quarks. The two most common modes of natural radioactive decay are alpha decay and beta decay. Most nuclear reactions emit energy in the class of gamma rays.
Vocabulary
- Blastoff decay - A common way of radioactive decay in which a nucleus emits an alpha particle (a helium-four nucleus).
- Beta decay - A mutual mode of radioactive disuse in which a nucleus emits beta particles. The daughter nucleus volition have a college atomic number than the original nucleus.
- Quark - Particles that form one of the two basic constituents of matter. Various species of quarks combine in specific ways to form protons and neutrons, in each example taking exactly iii quarks to make the composite particle.
Contributions & Attributions
This page was constructed from content via the following correspondent(southward) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Source: https://chem.libretexts.org/Courses/can/intro/17%3A_Radioactivity_and_Nuclear_Chemistry/17.03%3A_Types_of_Radioactivity%3A_Alpha%2C_Beta%2C_and_Gamma_Decay
Posted by: cliffordbutertench.blogspot.com


0 Response to "What Is The Change In The Atomic Number When An Atom Emits An Alpha Particle"
Post a Comment